Abstract
Background Patients (pts) with Acute Myeloid Leukemia (AML) have a bleak prognosis, particularly those who are elderly and/or have significant comorbidities. The treatment paradigm for such patients has shifted to less-intensive regimens, primarily venetoclax (Ven) plus either azacitidine (Aza) or decitabine (Dec). However, to date, it is unclear which regimen is optimal and which new molecular classifications are relevant. We performed a real-world analysis of our center's experience with Ven-based regimens to address the role of measurable residual disease (MRD) and the new ELN2022 in predicting clinical outcomes.
Methods We conducted a retrospective review of consecutive adult pts (aged ≥ 18 yo) at Roswell Park Comprehensive Cancer Center with a confirmed diagnosis of AML who received a Ven-based regimen between July 2017 and December 2021. Newly diagnosed (ND) and relapsed/refractory (R/R) AML pts were included. Demographics, disease-specific variables [including cytogenetics and mutations], and outcomes [responses, MRD status by multiparameter flow cytometry, and transplant status] were collected. Responses were defined according to the 2003 International Working Group criteria. For overall survival (OS), Kaplan-Meier method estimated the survival functions and log-rank test was used for between-group comparisons. Cox proportional hazards model was used for multivariable analysis. Univariate associations between categorical variables were assessed using Fisher's exact tests. Logistic regressions were used to evaluate the multivariable association between independent variables and treatment responses.
Results 83 pts (63 ND, 20 R/R) were analyzed. Median age was 73 years (range: 32-89); 62.7% were male. 50 pts (60%) received Aza/Ven, 27 Dec/Ven, and 6 (7%) LDAC/Ven. By ELN 2017 criteria, 42 (51.9%) pts had intermediate-risk cytogenetics while 37 (45.7%) had adverse-risk cytogenetics. Per ELN 2022 criteria, 11 (14.9%) had intermediate-risk disease while 58 (78.4%) had adverse-risk disease. Of note, 18 pts with favorable or intermediate-risk disease based on ELN 2017 criteria were re-classified as adverse-risk by ELN 2022 risk stratification, likely due to the addition of 7 mutations in the ELN 2022 risk stratification. 20 pts (24.1%) underwent allogeneic stem cell transplant (alloSCT) after receiving ven-based regimen.
Median OS (mOS) for all pts was 11 months (mos) (95% CI: 8-19.5 mos). No significant differences in mOS were observed with gender, age (<75 vs. ≥ 75 yo), or ELN 2017. ND AML pts had significantly improved mOS, vs R/R disease (13 vs. 5.5 mos; p=0.015). ELN 2022 adverse risk (p=0.01) and TP53 mutation (p=0.006) were associated with significantly poorer OS. Pts who underwent alloSCT after receiving ven-based therapy had improved mOS (19.5 vs. 8 mos; p=0.01). Although there was no statistically significant difference in OS due to regimen, there was a trend towards better mOS with Aza/Ven over Dec/Ven (11.3 vs 8 mos, p=0.17) in ND-AML, likely reflecting higher numbers of TP53 mutant pts in the latter group (11.4% vs 37.5%, p=0.025). There was no difference in OS based on ven-based regimens in secondary AML pts.
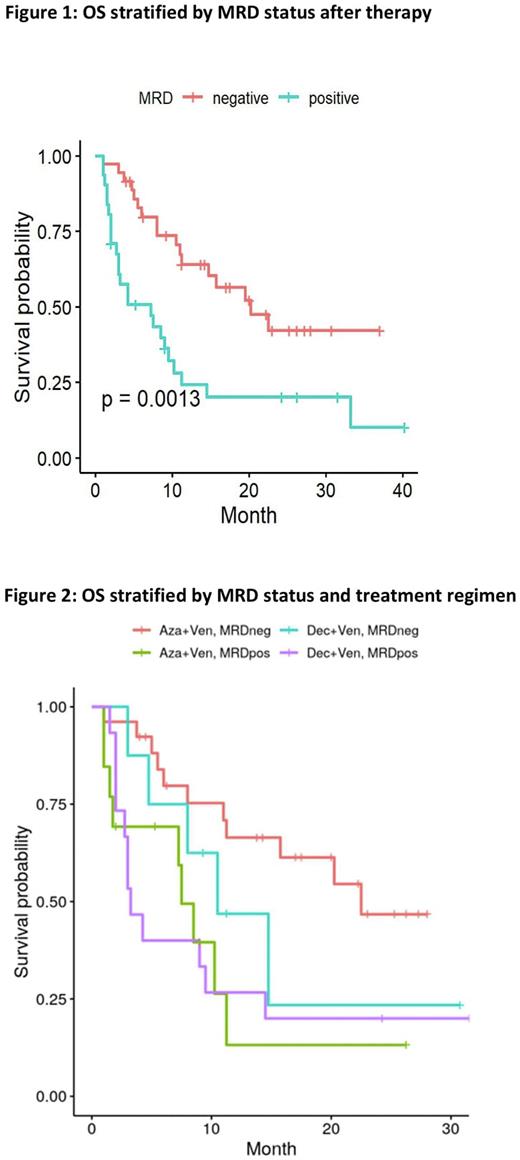
In the entire cohort, the CR/CRi rate was 43%: Aza/Ven 49% vs Dec/Ven 34.6% (p=0.33). In 67 pts with CR/CRi and MRD results, 36 (53.7%) achieved MRD negativity at any time point following therapy. MRD negativity was associated with improved mOS for all pts in univariate analysis (20.25 vs. 7.25 mos; p=0.0013) (Figure 1). MRD status also predicted for outcomes of either regimen (Figure 2). Pts treated with Aza/Ven demonstrated significantly higher rates of MRD neg CR/CRi than Dec/Ven (72.2% vs 22.2%; p=0.037). Multivariate Cox regression analysis demonstrated MRD status, ND vs R/R AML, alloSCT, and secondary AML (but not regimen) as independent factors for OS.
Conclusions In this real-world analysis, MRD-positive CR/CRi, TP53 mutation, and ELN 2022 adverse risk were associated with worse OS following Ven-based therapy. AML pts treated with Aza/Ven had higher rates of MRD-negative CR/CRi and a trend to improved OS vs Dec/Ven, possibly due to higher numbers of TP53 mutant pts receiving the latter therapy. Our results, while limited by small pt numbers, support wider adoption of MRD endpoints and improved molecular classification as predictors of outcomes of ven-based therapy. Further comparison of different hypomethylating agents with ven is also warranted.
Disclosures
Thompson:Novartis: Research Funding; Bristol Myers Squibb: Research Funding. Przespolewski:Jazz Pharmaceuticals: Research Funding. Griffiths:AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals: Research Funding; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Research Funding; Celldex Therapeutics: Research Funding; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Medicom Worldwide: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Physician Educational Resource: Honoraria; Picnic Health: Honoraria; Takeda Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Apellis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AAMDSIF: Honoraria. Wang:Kite Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Macrogenics: Consultancy; PTC Therapeutics: Consultancy, Honoraria, Other: Advisory Board; Novartis: Consultancy, Honoraria, Other: Advisory Board; Dava Oncology: Consultancy, Speakers Bureau; Rafael Pharmaceuticals: Other: Data Safety Monitoring Committee; Gilead: Consultancy, Honoraria, Other: Advisory Board; Stemline Therapeutics: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Pfizer: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Mana Therapeutics: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Abbvie: Consultancy, Honoraria, Other: member of data monitoring committee ; Kura Oncology: Consultancy, Honoraria, Other: Advisory Board, Steering Committee, Speakers Bureau; Daiichi Sankyo: Consultancy, Honoraria, Other: Advisory Board; Takeda: Consultancy, Honoraria, Other: Advisory Board; Genentech: Consultancy; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Other: Advisory Board; Astellas: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal